Your current status
- Necessary
- Analytics
- Targeting
Show details

Recently, Dr. Yuriy Abramov and Dr. Jian Wang from Porton’s team of global chemical engineering and technology published a Communication paper titled "Is It Salt, Cocrystal, or Continuum? Successes and Limitations of Computationally Efficient Periodic System Calculations" in Crystal Growth & Design, the American Chemical Society journal. This peer-reviewed journal focuses on publication of new research results in theoretical and experimental studies of the physical, chemical, and biological phenomena and processes related to the design, growth, and application of crystalline materials. The topic of this publication was invited to be shared at the 2024 Crystal Engineering Gordon Research Conference - a premier, international scientific conference focused on advancing the frontiers of science through the presentation of cutting-edge novel research.
Pharmaceutical crystals constitute the most prevalent commercial solid forms of small molecule drugs. If crystallization of a single drug molecule yields a solid form with undesirable properties (aqueous solubility, stability, mechanical properties, crystal morphology, etc.), a common strategy involves a solid form change from a single component to a multicomponent form. For acid−base combinations in multicomponent crystals, a spectrum of solid forms from salt to cocrystal may exist, characterized by the location of the proton (H+) between the acid and base molecules.
While both the cocrystal and salt forms of an active pharmaceutical ingredient (API) with a chosen conformer/counterion may result in significant improvements in the physical properties of the drug molecule, the definitive specification of the solid form of interest along the salt−cocrystal spectrum is crucial from regulatory and intellectual property perspectives. According to the latest FDA guidance, a cocrystal of the drug has a regulatory classification similar to that of a polymorph of the active pharmaceutical ingredient (API), whereas a new salt is treated as a new API.
Crystal structures of pharmaceutical salts and cocrystals are routinely determined through single-crystal X-ray diffraction (scXRD) experiments. However, it is important to recognize that X-rays are diffracted by electron density, and as a result, polar hydrogens exhibit the lowest scattering power relative to any other atom, while bare protons should be invisible. Therefore, the determination of proton location in the acid-base systems based solely on scXRD refinement should be approached with caution. Additional nonroutine experiments, such as single neutron diffraction (scND) or solid state nuclear magnetic resonance (ssNMR), may be required to confirm the proton location between acid and base. Such measurements may be time and cost consuming and are typically avoided in the basic pharmaceutical studies.
In the publication Dr. Yuriy Abramov and Dr. Jian Wang, proposed a practical (relatively fast and not expensive) computational workflow to support the characterization of pharmaceutical acid−base multicomponent crystals utilizing non-hydrogen atoms position obtained from scXRD. The proposed computational method was benchmarked against a data set of 24 acid-base crystallographic acid-base observations with proton positions experimentally assigned using ssNMR or scND methods.
It was demonstrated that if the experimentally observed N···O intermolecular hydrogen bond length is greater than ∼2.58 A, the acid−base structure can be 100% accurately and reliably determined by comparing the calculated energies of alternative salt and cocrystal configurations.
However, it was found that hydrogen bonds with N···O intermolecular distances shorter than ∼2.57 A have a high likelihood of forming a salt−cocrystal continuum configuration with shared proton between the acid and base, and cannot be reliably characterized by the computational approach.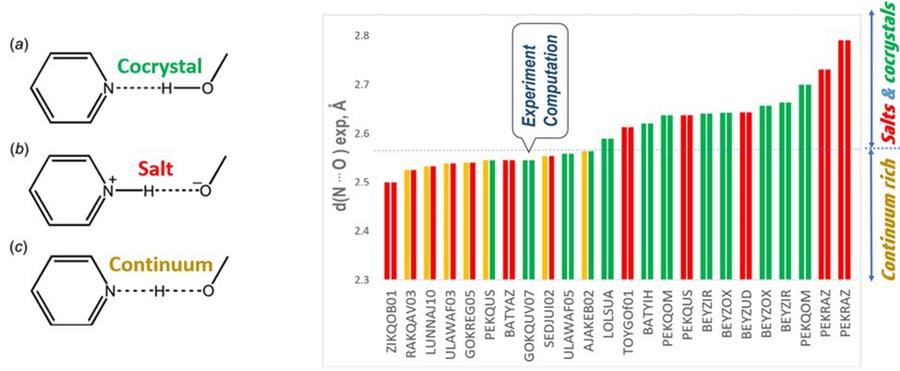
The proposed computational workflow is successfully applied to support Porton J-STAR projects at the request of clients.
About
Porton’s Global Chemical Engineering & Technology (GCET) center is a synergistic interdisciplinary technical platform for efficient support of process R&D and manufacturing of diverse drug substances (DS) and drug products (DP). The technical fields engaged in by GCET include Particle Engineering, Computational Chemistry & Data Science, Material Science & Engineering, Catalysis & Reaction Engineering, and Process Engineering. Led by diverse SMEs with rich experience from world leading pharma companies, the technical experts at GCET implement high-impact scientific and technical methodologies to address bottleneck problems in DS and DP process R&D and manufacturing, providing global drug development clients with high quality and efficient technical solutions.